Genetic Tuning
How It Works
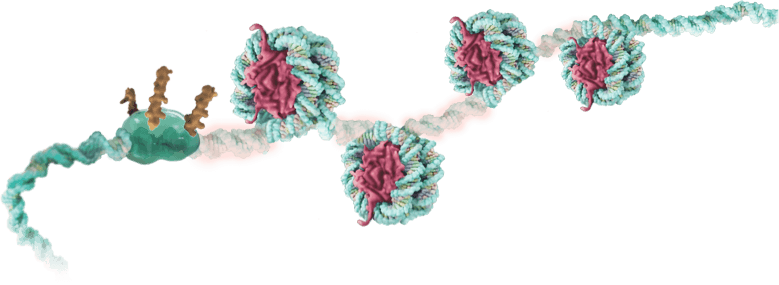
All cells contain the same DNA, but the epigenome determines how, when, and where genes are expressed.
These epigenomic changes drive the difference between nerve cells and muscle cells… between healthy, diseased, and exhausted cells.
The structures and signals of the epigenome can keep a gene packed tight, inaccessible, and silent…
…or wide open, accessible, and active within the cell.
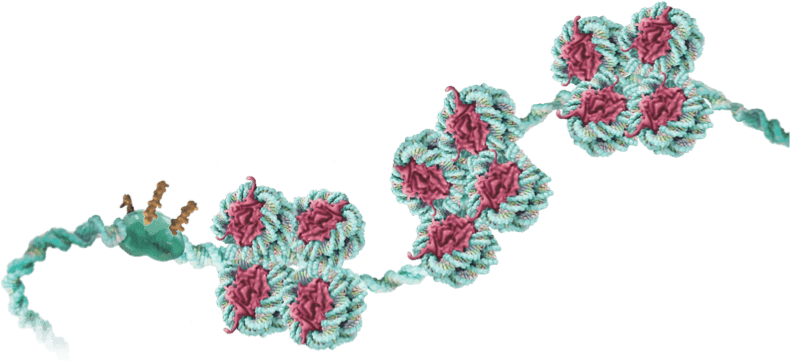
Our TEMPO genetic tuning platform works in concert with these master conductors of gene activity…
allowing us to turn up the volume on genes required for healthy cells and tissues…
…or turn down the volume on genes that cause or contribute to disease…
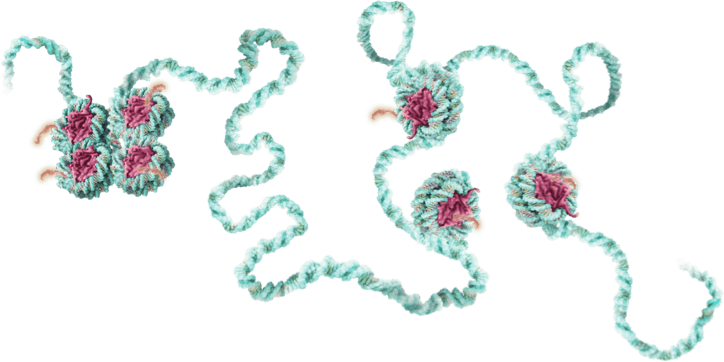
…ultimately rebalancing levels of gene expression – even in complex, multi-gene networks.
Genetic tuning via the epigenome opens the door to a powerful new class of treatments for common and chronic diseases.



The Science
Changing The Game
Epigenome editing is a unique modality of genetic medicine, enabled by cutting-edge advances in the understanding of gene regulation. The resultant technology enables exquisite levels of control over gene activity – activating, silencing, or fine-tuning the output of specific genes to reverse cellular dysfunction and disease.
Going Beyond the Code
Our TEMPO platform controls expression in single or multiple genes, by making targeted changes to DNA packaging and accessibility within cells. This is a fundamentally different process to gene editing, which creates cuts and breaks in DNA strands and makes irreversible changes to their fundamental coding sequence.

Enabling the Future
With a passionate commitment to exploration and innovation, Tune is driving through an exciting inflection point in the history of genetic medicine: from targeting a limited range of rare conditions to addressing thousands of both rare and common diseases for which no curative treatment is available. And in so doing, striving toward our ultimate goal: to unlock the full power and potential of regenerative medicine.

The Critical Difference
Where gene editing cuts or swaps DNA, genetic tuning leaves DNA sequences and naturally-encoded mechanisms of gene regulation intact – reducing disruption and providing more fine-tuned, predictable control within complex gene networks.
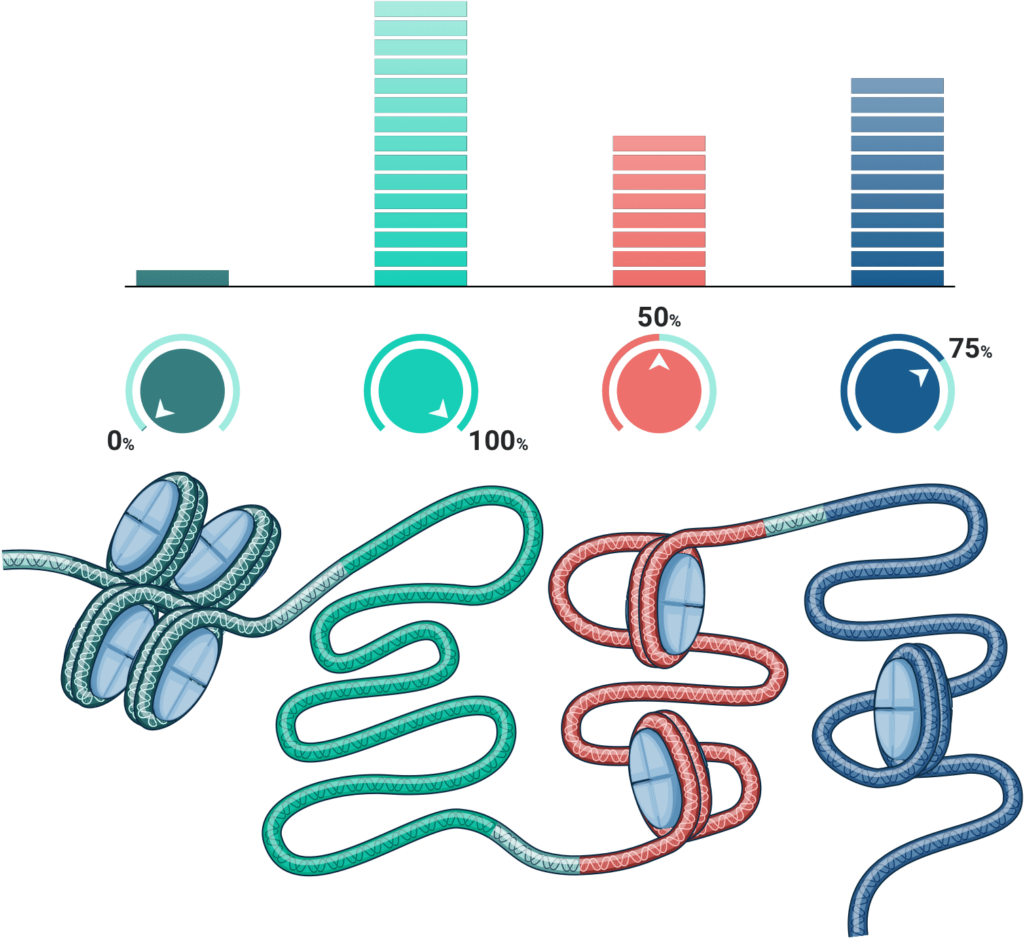
And critically, because genetic tuning does not create strand breaks in DNA, it is far better suited to working with multiple genes at once (or multiplexing) when compared to other gene therapy modalities.

Across all technologies that alter DNA sequence, any nicking, cutting, or breaking of DNA is always associated with the risk of making unintended changes to the genome at off-target locations. Attempting to target two or more sites multiplies this risk. In short, the more DNA edits you try to make, the greater the potential for variable and unpredictable results.

By contrast, genetic tuning not only provides exquisite target specificity, it also rules out the possibility of off-target DNA damage and repair responses – because no damage is done, and no repair is necessary.
Armed with this technology, we can confidently target not just single genes, but multiple nodes of control within a complex gene network. This expands the scope of genetic tuning to complex diseases involving the interaction of multiple genes and cell types – which describes the vast majority of common and chronic diseases we face in the modern world.
Power and Potential
With the power to target multiple genes and gene networks at once, genetic tuning opens the door to a powerful new possibility in medicine – the ability to shift cell type, state, and function at will.
This follows from two self-evident biological truths:
- 1
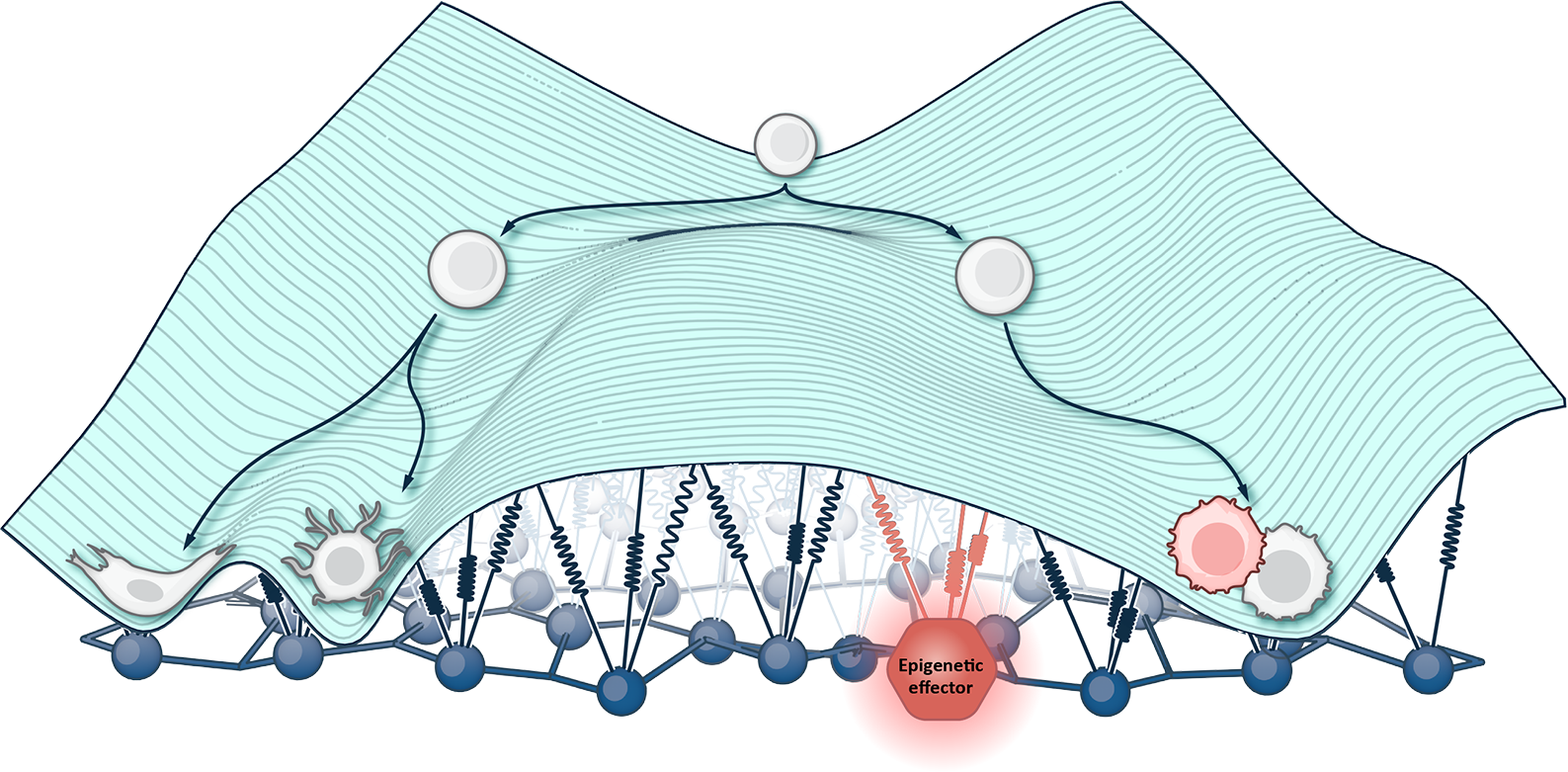
- All cells types have the same DNA. It is the differences in their epigenetics, not their basic code, that makes them distinct. With enough understanding and control of the epigenetic mechanisms that differentiate stem cells into mature nerve, muscle, or blood cells, you can theoretically turn any given cell type A into any desired cell type B.
- 2
- Almost all complex diseases involve a shift in epigenetic cell state during progression. These epigenetic changes trigger and reinforce pathways of cell exhaustion, loss of function, and cell death. Figure out how to reverse these states, and you hold the potential to reverse pathways of disease – including rare monogenic diseases, common tumor development, degenerative brain diseases, and the tissue-specific effects of aging.
This is what we are so excited about at Tune. We are leveraging the awesome power of epigenomic control to create the next generation of genetic therapies. Through our unique therapeutic modality and platform, we believe we can address both rare and common diseases while unlocking the full power and potential of regenerative medicine.

